Heart group - Pr Bart Staels

Presentation of the research topic
As in many areas of patient care, the move away from a ‘one size fits all’ approach to an individualised ‘precision medicine’ approach represents the near and reasonably achievable future of cardiovascular disease treatment. The final goal of personalised cardiac medicine is to deliver optimised medical care and outcomes for each people, providing the best therapy for each patient in terms of efficacy, safety and reduction of associated costs.
Our research activities are therefore dedicated to personalised medicine to prevent and target cardiovascular disease through:
- New imaging biomarkers,
- DNA sequencing of circulating and myocardial leukocytes,
- Phenotyping of myocardial immunometabolism,
- Quantification (using GFET aptamer sensors) of cardiac biomarkers in biological samples (blood, saliva, sweat, exhaled air) from patients, where levels are very low or currently undetectable.
Our translational research, which extends from rodent models to patients, aims to improve understanding of the pathophysiology of cardiac remodelling, to refine cardiovascular risk stratification and the therapeutic strategy associated with benefit/risk, not only in patients with a history of cardiovascular disease, but also in patients with cardiovascular risk factors such as smoking, type 2 diabetes, dyslipidaemia and obesity, even in the absence of any cardiovascular medical history or symptoms.
Our projects focus more specifically on cardiac function, energy metabolism and remodelling in patients with diabetes and/or heart valve disease, and aim to improve patient stratification and develop personalised cardiovascular medicine.
If you need more information, click here
Key-Words
Cardiac remodelling ; valvulopathies ; arrhythmia ; atrial fibrillation ; energy metabolism ; inter-organ communication ; immuno-metabolism ; cardiac imaging.

COISNE Augustin
PU-PH - Cardiology
ORCID : 0000-0002-1662-7874
Pr Augustin COISNE (MD, PhD, HDR) is a cardiologist (medical thesis in 2013). He has held the position of Professor of Cardiology at the Lille Faculty of Medicine (University of Lille, France) since 2023. He obtained his doctorate in biological sciences (PhD) in 2018 (Lille University) and is qualified to direct research (HDR in 2021). He joined the INSERM UMR1011 unit, headed by Professor Bart Staels, in 2013. In this team, Augustin Coisne is involved in ‘cardiovascular translational research’ projects focusing on cardiac function, metabolism and remodelling in patients suffering from diabetes and/or valvular heart disease.
Prof. Augustin Coisne has also been head of the cardiology unit of the Cardiovascular Physiology & Functional Explorations (EFCV) department at Lille University Hospital since 2018. He completed a post-doctoral fellowship at the Clinical Trial Center and Skirball Center for Innovation of the Cardiovascular Research Foundation in New York, NY, USA in 2021 (Dr Juan Granada).
He is also coordinator of the Valvulopathy Research and Innovation Committee at Lille University Hospital. He has co-authored around a hundred articles, mainly in the field of translational and clinical research into valve disease and imaging, with first-author publications in the European Heart Journal, JAMA Cardiology, JACC, JACC Cardiovascular Intervention, JACC Cardiovasc Imaging and Heart.
He is a member of the board of the Cardiovascular Imaging Branch of the Société Française de Cardiologue, a former French ambassador for the Heart Imagers of Tomorrow (HIT) programme of the European Association of Cardiovascular Imaging (EACVI), a member of the SFC Valves Group and the PCR tricuspid focus group.

PONTANA François
Fonction PU-PH – Radiologist and medical imaging
francois.pontana[@]chu-lille.fr
ORCID : 0000-0001-5316-7101

LONGERE Benjamin
MCU-PH – Radiologist and medical imaging
benjamin.longere@chu-lille.fr
ORCID : 0000-0002-0340-5404

LEMESLE Gilles
PU-PH - Cardiology
gilles.lemesle[@]chu-lille.fr
ORCID : 0000-0002-2917-9273
Heart-Lung Institute - Department of Cardiology - Cardiac Intensive Care Unit and Catheterisation Laboratory - Lille University Hospital - FRANCE.
Gilles Lemesle, is an academic cardiologist (medical thesis in 2007) on a full professorship in cardiology at the Lille Medical School since 2017. He completed a fellowship in Washington DC in 2008-2009 (Pr Ron Waksman and Augusto Pichard, Washington Hospital Center, USA). He obtained his doctorate in biological sciences (PhD) in 2015 and is qualified to direct research (HDR in 2016). He joined the INSERM UMR1011 unit, headed by Pr Bart Staels, in 2016. In this team, Gilles Lemesle leads ‘cardiovascular translational research’ projects focusing on myocardial infarction, coronary artery disease and the management of antithrombotic treatments in coronary patients with or without diabetes. Gilles Lemesle has also been head of the cardiology emergency and intensive care unit at Lille University Hospital since 2022.
He is co-author of more than 200 articles and has published around 40 articles as first author in the field of translational and clinical research, focusing on myocardial infarction, coronary disease and the management of antithrombotic treatments.
In this capacity, he is coordinator of randomised studies and principal investigator of numerous trials.
He has been a member of the GRRC group (Groupe de Réflexion en Recherche Cardiovasculaire, FRANCE) since 2013 and has been the group's chairman since 2024. He has also been a member of the FACT group (French Alliance for Cardiovascular Trials, FRANCE) since 2016. He is a member of the SFC (Société Française de Cardiologie), the GACI (Groupe Athérome Coronaire et Cardiologie Interventionnelle), the ESC (European Society of Cardiology) and the EAPCI (European Association of Percutaneous Cardiovascular Intervention).

NINNI Sandro
MCU-PH – Cardiologst
sandro.ninni[@]chu-lille.fr
ORCID : 0000-0001-6179-6098
Sandro Ninni is an academic cardiologist (medical thesis in 2016) with a post as Associate Professor of Cardiology at the Lille Medical School (University of Lille, France) since 2020. He obtained his doctorate in biological sciences (PhD) in 2021 and his habilitation to direct research (HDR) in 2024. He joined the INSERM UMR1011 unit, headed by Professor Bart Staels, in 2017. In this team, Sandro NINNI has developed ‘cardiovascular translational research’ projects focusing on cardiac arrhythmia and its association with inflammatory response and metabolic disorders.
Sandro NINNI is also involved in the Department of Cardiovascular Medicine, with specific expertise in cardiac arrhythmias and cardiac electrophysiology. Sandro NINNI is also doing a post-doctoral fellowship in Montreal (Montreal Heart Institute, Dr Nattel's laboratory) to develop new skills in the field of cellular electrophysiology.
He has published articles in the field of cardiac (patho)physiology and cardiac arrhythmias. He has been an elected member of the scientific committee of the Société Française de Cardiologie: Groupe de Réflexion sur la Recherche Cardiovasculaire since 2022.

TAZIBET Amine
Cardiologist - PhD Student in sciences
amine.tazibet[@]chu-lille.fr
ORCID : 0009-0007-5564-6625
Amine Tazibet is a young cardiologist (medical thesis in 2022) specialising in rhythmology and cardiac stimulation. He has been studying for a PhD in Science since 2023, with a thesis focusing on the evaluation of atrial remodelling and the inflammatory profile of patients, in particular clonal haematopoiesis. He is aiming for a career in a university hospital, with an initial position as Assistant Head of Clinic at Lille University Hospital from November 2025.
DUBRULLE Henri
Cardiac surgeon - PhD Student in sciences
henri.dubrulle@chu-lille.fr

WOITRAIN Eloïse
Engineer
eloise.woitrain[@]pasteur-lille.fr
ORCID : 0000-0002-9913-9023
Eloïse Woitrain is an engineer at Lille University Hospital. She obtained her Master's degree in Biology and Health from the University of Lille in 2013.
At the end of her studies, she joined the INSERM UMR1011 unit, headed by Professor Bart Staels. Eloïse first joined the team working on metabolic liver pathologies for 5 years. She focused mainly on exploring new drugs to treat MASH using in vivo and in vitro models to test the efficacy and safety of these treatments. In 2018, Eloïse joined Prof David Montaigne's team and is involved in translational research projects focusing on cardiac metabolism and remodelling in patients with valvulopathy and/or diabetes. At the same time, she is involved in research projects focusing on cardiac remodelling caused by metabolic diseases, using preclinical and clinical models.

BUTRUILLE Laura
Postdoctoral researcher
laura.butruille[@]pasteur-lille.fr
ORCID : 0000-0001-5760-1489
Laura Butruille is a postdoctoral researcher in the field of metabolic and cardiovascular pathologies. She defended her PhD in Physiology in 2013 at the University of Lille. After a brief stint in a biomedical start-up (Mdoloris Medical Systems), she joined Prof. Laurent Storme's team to identify perinatal mechanisms that could explain adult metabolic diseases. In 2018, she joined the INSERM UMR1011 unit, headed by Prof Bart Staels, and has been working in Prof David Montaigne's group since 2020. She is developing research projects focusing on cardiac remodelling induced by metabolic diseases such as MASLD/MASH in preclinical and clinical models.

WITOCKX Kimberly
Assistant engineer
kimberly.witockx[@]univ-lille.fr
ORCID : 0009-0003-9981-8127
Kimberly WITOCKX is an assistant engineer. She has a professional degree in therapeutic innovations and biotechnologies. After a short spell at LFB in Lille (Laboratoire français du fractionnement et des biotechnologies), she continued her career by joining Cerballiance, a medical analysis laboratory. In 2023, she joined the INSERM UMR1011 unit, headed by Professor Bart Staels, and joined Professor David Montaigne's group. She is involved in in vitro and in vivo experiments at both preclinical and clinical levels.
We demonstrated in 2018 that time of day affects patients' cardiac outcomes with more severe consequences when surgical aortic valve replacement was performed in the morning compared to the afternoon (Montaigne D, et al., Lancet. 2018).
Through collaboration with members of the Leducq consortium team, we investigated the specificity of clonal haematopoiesis (CHIP) patients undergoing cardiac surgery. We showed that these patients had a significantly higher rate of postoperative atrial fibrillation and an increased risk of major cardiovascular complications (cardiovascular death, stroke, hospitalisation for acute heart failure, etc.) (Ninni S. et al., J Am Coll Cardiol. 2023).
In collaboration with Dr Jérémy Fauconnier of Montpellier, we have identified mitochondrial calcium homeostasis, and more specifically the mitochondrial calcium uniporter complex (MCUC), as a determinant of atrial fibrillation in metabolic syndrome and an attractive therapeutic target (Fossier L. et al., J Am Coll Cardiol 2022).
Our team has developed expertise in the following areas:
- Cardiac imaging (ultrasound, MRI)
- In vivo experiments (models of aortic constriction, telemetry, transoesophageal atrial stimulation, etc.)
- In vitro experiments (cell culture)
- Study of the cardiac phenotype (histology, gene and protein expression, cardiomyocyte energy metabolism, inflammation markers using multiplexing techniques)
- Analysis of mitochondrial function (Oroboros, Sea Horse)
- Immunophenotyping (FACS / single cell RNAseq)
List of mainspublications by the heart group of team 1:
Coisne A, Montaigne D, Aghezzaf S, Ninni S, Lemesle G, Sudre A, Lamblin N, Modine T, Vincentelli A, Juthier F, Leon MB, Granada JF, Bauters C. Clinical Outcomes According to Aortic Stenosis Management: Insights From Real-World Practice. J Am Heart Assoc. 2024 Nov 19;13(22):e036657. doi: 10.1161/JAHA.124.036657. Epub 2024 Nov 15. PMID: 39548024; PMCID: PMC11681392.
Aghezzaf S, Coisne A, Bauters C, Favata F, Delsart P, Coppin A, Seunes C, Schurtz G, Verdier B, Lamblin N, Tazibet A, Le Taillandier de Gabory J, Ninni S, Donal E, Lemesle G, Montaigne D. Feasibility and prognostic significance of ventricular-arterial coupling after myocardial infarction: the RIGID-MI cohort. Eur Heart J Cardiovasc Imaging. 2024 Apr 30;25(5):668-677. doi: 10.1093/ehjci/jead342. PMID: 38133627.
Ninni S, Dombrowicz D, de Winther M, Staels B, Montaigne D, Nattel S. Genetic Factors Altering Immune Responses in Atrial Fibrillation: JACC Review Topic of the Week. J Am Coll Cardiol. 2024 Mar 26;83(12):1163-1176. doi: 10.1016/j.jacc.2023.12.034. PMID: 38508850.
T. Rodrigues, V. Mishyn,Y. R. Leroux, L. Butruille, E. Woitrain, A. Barras, P.Aspermair, H. Happy, C. Kleber, R. Boukherroub, D. Montaigne, W. Knoll, S. Szunerits (2022). Highly performing graphene-based field effect transistor for the differentiation between mild-moderate-severe myocardial injury
. Nano Today, 2022 doi.org/10.1016/j.nantod.2022.101391
Montaigne D, Butruille L, Staels B. PPAR control of metabolism and cardiovascular functions. Nat Rev Cardiol. 2021 Dec;18(12):809-823. doi: 10.1038/s41569-021-00569-6. Epub 2021 Jun 14. PMID: 34127848. (Review)
Montaigne D, Marechal X, Modine T, Coisne A, Mouton S, Fayad G, Ninni S, Klein C, Ortmans S, Seunes C, Potelle C, Berthier A, Gheeraert C, Piveteau C, Deprez R, Eeckhoute J, Duez H, Lacroix D, Deprez B, Jegou B, Koussa M, Edme JL, Lefebvre P, Staels B. Daytime variation of perioperative myocardial injury in cardiac surgery and its prevention by Rev-Erbα antagonism: a single-centre propensity-matched cohort study and a randomised study. Lancet. 2018 Jan 6;391(10115):59-69. doi: 10.1016/S0140-6736(17)32132-3. Epub 2017 Oct 26. PMID: 29107324.
Montaigne D, Marechal X, Coisne A, Debry N, Modine T, Fayad G, Potelle C, El Arid JM, Mouton S, Sebti Y, Duez H, Preau S, Remy-Jouet I, Zerimech F, Koussa M, Richard V, Neviere R, Edme JL, Lefebvre P, Staels B. Myocardial contractile dysfunction is associated with impaired mitochondrial function and dynamics in type 2 diabetic but not in obese patients. Circulation. 2014 Aug 12;130(7):554-64. doi: 10.1161/CIRCULATIONAHA.113.008476. Epub 2014 Jun 13. PMID: 24928681.
Montaigne D, Marechal X, Lefebvre P, Modine T, Fayad G, Dehondt H, Hurt C, Coisne A, Koussa M, Remy-Jouet I, Zerimech F, Boulanger E, Lacroix D, Staels B, Neviere R. Mitochondrial dysfunction as an arrhythmogenic substrate: a translational proof-of-concept study in patients with metabolic syndrome in whom post-operative atrial fibrillation develops. J Am Coll Cardiol. 2013 Oct 15;62(16):1466-73. doi: 10.1016/j.jacc.2013.03.061. Epub 2013 May 1. PMID: 23644086.
List of the different projects and funding of the core group of team 1 :
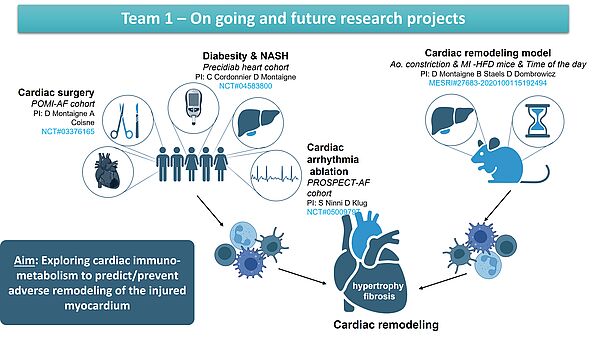
1- TOMIS and POMI-AF clinical project funded by the “Agence Nationale pour la Recherche” young researcher grant (ANR TOMIS-Leukocyte: ANR-CE14-0003-01) and the Fondation Leducq LEAN 16CVD01 network. The project is dedicated to better understanding post-operative complications (e.g. myocardial infarction, heart failure and atrial fibrillation) after cardiac surgery, with a particular interest in understanding the impact of the time of day of surgery on outcomes.
2- Preci-Diab Heart clinical project: as part of the National Center for Precision Diabetic Medicine www.precidiab.org and funded by the French National Research Agency (ANR-18-IBHU-0001). The project is dedicated to improving patient phenotyping using new biomarkers and cardiac imaging in order to adapt personalized cardiac medicine for type 2 diabetes patients.
3- RIGID-MI project (PI Prof. G Lemesle) This clinical project is dedicated to a better understanding of cardiac remodelling and complications (e.g. myocardial infarction, heart failure and atrial fibrillation) following a myocardial infarction.
4- VALVENORD project (PI Prof. C Bauters) This clinical project aims to gain a better understanding of remodelling and cardiac complications (e.g. myocardial infarction, heart failure and atrial fibrillation) in outpatients with aortic valve stenosis (mild to severe) in the Haut de France region. The project is funded by the “Fédération Française de Cardiologie”.
5- The CALMOS translational project (coll. with J Fauconnier Univ Montpellier) is funded by a grant from the Agence Nationale pour la Recherche (ANR CALMOS: ANR 18-CE17-0003-02). This project is dedicated to better understanding the role of cardiac mitochondria in cardiac arrhythmias.
6- The LivImmCar translational project aims to highlight the interaction of hepatic steatosis associated with metabolic dysfunction (MASH) and its immune alterations with cardiac remodelling in patients suffering from cardiovascular disease. The project is funded by the Fédération Française de Cardiologie and the Fondation pour la Recherche Médicale
Pr MONTAIGNE David
PU-PH de Cardiologie, group leader of "Heart group" in Team 1