Topic 7 : ENDO-PLAST - Role of endothelial cell plasticity and metabolic reprogramming in diseases , Emerging Atip-Avenir team

Presentation of the research topic
The endothelium plays an indispensable role in all tissues, and its dysfunction contributes to a variety of cardiovascular disorders, including atherosclerosis, as well as cancer. Targeting dysfunctional blood vessels represents a promising avenue for the development of therapies aimed at preventing or treating the most prevalent diseases.
Our research aims at characterizing the role of endothelial cell (EC) dysfunction in the onset and progression of cardiovascular diseases and cancer. Specifically, we focus on EC plasticity and associated metabolic reprogramming as drivers of disease progression.
By combining multidisciplinary expertise and using advanced cellular and molecular approaches, as well as in vivo transgenic mouse models, our ENDO-PLAST team strives to decipher the complex mechanisms regulating the pathological transdifferentation of ECs into mesenchymal-like cells (endothelial-to-mesenchymal transition, EndMT) with the ultimate goal to identify novel means to target this process. Our work is part of a translational approach aiming, in the long-term, at translating our discoveries into clinical applications to improve the care of patients suffering from cardiovascular diseases and cancer.
Key-words
Vascular biology; endothelial cells; endothelial plasticity; vessel permeability; metabolism; metabolic heterogeneity.
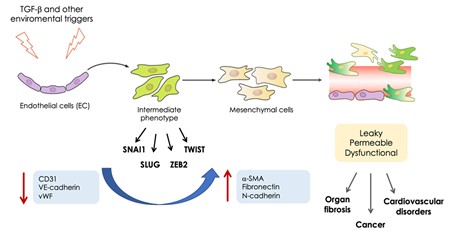
EndMT process: Following environmental stimuli, including TGF-b, hypoxia and oxidative stress, ECs progressively lose their identity by downregulating the canonical endothelial markers (CD31, VE-cadherin, von Willebrand factor) and acquiring the expression of mesenchymal markers (a-SMA, fibronectin, N-cadherin) and transcription factors (SNAI1, SLUG, ZEB2, TWIST) leading to dysfunctional vessels.

Anna Rita Cantelmo
Team leader - CRCN Inserm scientist
anna-rita.cantelmo[@]univ-lille.fr
I began my scientific journey by pursuing a PhD in Dr. Adriana Albini's lab at MultiMedica, Milan (Italy), where I focused on developing innovative strategies to target tumor angiogenesis. After my PhD, I joined the lab of Prof. Peter Carmeliet at VIB-KUL, Leuven (Belgium), where I explored the role of the glycolytic regulator PFKFB3 in endothelial cell metabolism and its impact on angiogenesis and cancer progression. This research resulted in significant publications that enhanced my expertise in vascular biology. In 2018, I relocated to France, where I obtained funding to pursue independent research on angiogenesis at the University of Lille. I am a recipient of the prestigious Atip-Avenir program, and my team is based within UMR1011 at the Institut Pasteur de Lille, where we integrate basic and translational research to investigate how EC abnormalities contribute to cardiovascular diseases.
Cyril Robil, MSc
Senior Engineer
cyril.robil[@]pasteur-lille.fr
I am an engineer working at the Institut Pasteur de Lille since 2020. I hold a Master’s degree in Biotechnology from the University of Lille and a Bachelor’s degree in Biology/Biochemistry from the University of Orléans. My previous research focused on COVID-19-related projects, including the study of cellular senescence and the role of the gut microbiota in disease development. During this period, I managed multiple research projects and participated in several scientific collaborations. Prior to joining the Institut Pasteur de Lille, I worked as technician at GENFIT, where I contributed to the development of therapies for liver diseases, specializing in proteomic and transcriptomic analyses. Within the team led by Dr. Anna Rita Cantelmo, I support ongoing projects through experimental planning, technical coordination, and resource management. I am involved in experimental design and execution, protocol optimization, data analysis, and supervision and training of junior staff, while also coordinating day-to-day laboratory operations, including shared resources, reagent ordering, and compliance with safety and quality standards.
Oksana Plu, MSc
Engineer
oksana.plu[@]pasteur-lille.fr
After obtaining a Bachelor’s degree in Chemistry, I graduated from the Université de Picardie Jules Verne with a Master’s degree in Biology and Health, specializing in Microbiology and Oncology. During my internships, I worked on tumor heterogeneity in bladder cancer, as well as on the role of Lactobacillus species in inflammation and in the colonization by Candida albicans. These experiences enabled me to develop strong technical expertise in microbiology, molecular and cell biology, and histological techniques. In October 2025, I joined the research team led by Dr. Anna Rita Cantelmo as a research technician. In this role, I support multiple research projects, including those focusing on the mitochondrial protein CHCHD4 and EndMT. I contribute to the implementation and optimization of experimental protocols, provide technical support for both in vitro and in vivo studies, and participate in data acquisition and analysis.

Louay Bettaieb, PhD
Postdoctoral researcher
My research focuses on exploring how ECs reprogram their metabolism during disease progression. I completed both my MSc and PhD at the University of Lille within the research team led by Dr. Anna Rita Cantelmo, culminating in the award of my PhD in 2025. My project investigates the role of the mitochondrial protein import factor CHCHD4 in angiogenesis and its contribution to cardiovascular diseases. My work combines advanced molecular and cellular biology approaches with in vivo mouse models to dissect how CHCHD4-dependent mitochondrial regulation controls endothelial plasticity and vessel function. Through interactions with translational research teams, I aim to bridge fundamental mechanisms with therapeutic strategies targeting mitochondrial dysfunction and vascular abnormalities, ultimately contributing to improved patient outcomes.

Amani Machmouchi, MSc
PhD student, University of Lille
amani.machmouchi[@]univ-lille.fr
Passionate about biology and medical research, I obtained a Bachelor’s degree in Biology from the Lebanese University, followed by a Bachelor’s degree in Animal Biology and a Master’s degree in Research in Immunology. I also pursued additional training in Health Administration. I am currently a PhD student within UMR1011 at the Institut Pasteur de Lille, working on the project "Endothelial-to-Mesenchymal Transition (EndMT) in pathological angiogenesis," with a particular focus on atherosclerosis and cancer. My research aims to uncover the mechanisms driving EndMT and to understand its contribution to vascular remodeling and disease progression. My work integrates in vivo mouse models, including endotracer-based approaches, with cellular and molecular techniques to study EndMT in pathological settings. In parallel, I integrate bioinformatics approaches, including meta-analysis of publicly available transcriptomic datasets, to establish a robust and conserved EndMT gene signature across different pathological models. By combining mechanistic studies with disease-relevant models, my goal is to identify and exploit novel EndMT drivers to limit vascular dysfunction in atherosclerosis and related cardiovascular diseases.

Evan Courmont, MSc
PhD student, University of Lille (in co-supervision with Prof. Dimitra Gkika, CANTHER)
evan.courmont.etu[@]univ-lille.fr
Fascinated by science, I completed a BSc in Cellular Biology and Physiology in 2021 and obtained my MSc in Biology and Health (Oncology section) in 2023 at the University of Lille. Since my Master training, I have been working in the research team led by Dr. Anna Rita Cantelmo, where I initially explored the role of nuclear receptors in ECs and vascular functions. I am currently pursuing my PhD in co-supervision with Dimitra Gkika at CANTHER laboratory (UMR9020 CNRS / UMR1277 Inserm). My work combines molecular and cellular biology approaches with functional assays and disease-relevant models to dissect how calcium-dependent signaling pathways control endothelial plasticity, cell fate decisions, and tissue remodeling. By integrating vascular biology with ion channel research, my goal is to identify novel mechanisms and therapeutic targets to limit EndMT-driven vascular and fibrotic diseases.
Corina Birzoi, MSc
PhD student, University of Lille
corina.birzoi[@]pasteur-lille.fr
After completing a Bachelor’s in Molecular and Cellular Biology and a Master’s degree in Omics and Systems Biology at the University of Lille, I carried out an internship focused on sarcoma research, where I gained hands-on experience in advanced mass spectrometry techniques. I am currently pursuing a PhD project entitled “The role of the mitochondrial protein CHCHD4 in EC function”. This project investigates how CHCHD4-mediated regulation of mitochondrial homeostasis in ECs contributes to angiogenic remodeling in the context of systemic metabolic disorders. My work specifically aims to decipher how CHCHD4-dependent metabolic reprogramming modulates endothelial behavior, vascular integrity, and adaptive angiogenic responses under pathological conditions. Using a multidisciplinary strategy that combines in vitro mechanistic approaches with in vivo mouse models of metabolic disease, I investigate how endothelial-specific perturbation of the CHCHD4 pathway impacts angiogenic signaling networks, mitochondrial function, and vascular remodeling associated with metabolic dysfunction.
Recent publications:
Courmont E, Cantelmo AR. Calcium Signaling and Metabolic Reprogramming in Cancer: Mechanisms and Therapeutic Implications. Cold Spring Harb Perspect Biol. 2025.
Calcium signaling tightly couples metabolic reprogramming to cancer progression by reshaping mitochondrial function, redox balance, and cell fate decisions. This work highlights calcium-dependent metabolic plasticity as a central mechanism driving tumor growth and therapy resistance, and discusses its potential as a therapeutic vulnerability.
Lebas M, Chinigò G, Courmont E, Bettaieb L, Machmouchi A, Goveia J, Beatovic A, Van Kerckhove J, Robil C, Angulo FS, Vedelago M, Errerd A, Treps L, Gao V, Delgado De la Herrán HC, Mayeuf-Louchart A, L'homme L, Chamlali M, Dejos C, Gouyer V, Garikipati VNS, Tomar D, Yin H, Fukui H, Vinckier S, Stolte A, Conradi LC, Infanti F, Lemonnier L, Zeisberg E, Luo Y, Lin L, Desseyn JL, Pickering JG, Kishore R, Madesh M, Dombrowicz D, Perocchi F, Staels B, Pla AF, Gkika D, Cantelmo AR. Integrated single-cell RNA-seq analysis reveals mitochondrial calcium signaling as a modulator of endothelial-to-mesenchymal transition. Sci Adv. 2024.
EndMT, essential in embryonic development and linked to cardiovascular diseases, remains complex to modulate. Using single-cell RNA sequencing, we identified EndMT conserved gene signatures and found that mitochondrial calcium uptake is a key driver of EndMT. Inhibiting the mitochondrial calcium uniporter (MCU) prevented EndMT in vitro, and MCU deletion blocked mesenchymal activation in a disease model. Elevated MCU levels were also found in patients with critical limb ischemia. These findings suggest MCU as a potential therapeutic target for treating EndMT-related diseases.
Team news:
Congrats to Louay on successfully defending his PhD thesis. A big milestone for him and also for our team, as Louay is our very first PhD graduate! Well done, Louay - all the best for the next steps of your scientific journey, which will continue a bit longer with us (Dec 2025).
Amani will soon be leaving for a short research visit to Germany, where she will join the laboratory of Prof. Lena Conradi at the University of Göttingen to acquire expertise in human organoids. Many thanks to the Precision Health Program of the École Doctorale Biologie Santé Université de Lille for the financial support (Nov 2025).
Bravo to our students for their participation in the first edition of the IPL PhD Students’ Day, and in particular to Evan for presenting our work on EndMT in fibrosis (Nov 2025).
Our students Amani and Evan will participate to the Fête de la Science, actively engaging with the public to present our research activities and to promote scientific curiosity and dialogue. Have fun! (Oct 2025).
Our postdoc Mauro, along with other members of the unit participated in the Journée Européenne du Patrimoine. They explained the role of a researcher and the main research axes of UMR1011 to lay public (Oct 2024).
Our PhD student Louay Bettaieb will be soon spending three months in Leuven, Belgium, during his PhD mobility period. He will be joining the laboratory of Prof. Peter Carmeliet at VIB-KUL to gain additional vascular and metabolic expertise and generate final data for the completion of his PhD work (Sept 2024).
Our team has strong expertise in vascular biology, with a particular focus on the characterization of EC (dys)functions in physiological and pathological conditions. We have established protocols to isolate primary ECs from human umbilical cords collected from donors at the CHU Lille, enabling the generation of primary EC cultures for in vitro functional studies. In parallel, we have developed multiple transgenic mouse lines that allow us to study EC dynamics and plasticity in vivo, both under homeostatic conditions and in disease models. Our expertise includes the use of lineage tracing approaches, inducible Cre-lox systems, and disease-relevant models of cardiovascular and cancer-associated pathologies. At the molecular level, the team is skilled in advanced omics approaches, including bulk and single-cell RNA sequencing, integrative data analysis, and functional validation of candidate pathways. We are currently expanding our expertise in spatial transcriptomics and have received dedicated training on the cutting-edge Visium HD pipeline from 10x Genomics, enabling high-resolution spatial mapping of endothelial heterogeneity within complex tissues.
Fundings:
We are very grateful to all the funding agencies for their support of our work.

Publications:
Lebas M*, Chinigò G*, Courmont E*, Bettaieb L*, Machmouchi A, Goveia J, Beatovic A, Van Kerckhove J, Robil C, Angulo FS, Vedelago M, Errerd A, Treps L, Gao V, Delgado De la Herrán HC, Mayeuf-Louchart A, L’homme L, Chamlali M, Dejos C, Gouyer V, Garikipati VNS, Tomar D, Yin H, Fukui H, Vinckier S, Stolte A, Conradi LC, Infanti F, Lemonnier L, Zeisberg E, Luo Y, Lin L, Desseyn JL, Pickering J, Kishore R, Madesh M, Dombrowicz D, Perocchi F, Staels B, Pla AF, Gkika D, Cantelmo AR.
Integrated single-cell RNA-seq analysis reveals mitochondrial calcium signaling as a modulator of endothelial-to-mesenchymal transition
Sci Adv. 2024 Aug 9;10(32)
Dejos C, Gkika D, Cantelmo AR.
The Two-Way Relationship Between Calcium and Metabolism in Cancer.
Front. Cell Dev. Biol., 2020 Nov 13;8:573747.
Veys K, Fan Z, Ghobrial M, Bouché A, García-Caballero M, Vriens K, Conchinha NV, Seuwen A, Schlegel F, Gorski T, Crabbé M, Gilardoni P, Ardicoglu R, Schaffenrath J, Casteels C, De Smet G, Smolders I, Van Laere K, Abel ED, Fendt SM, Schroeter A, Kalucka J, Cantelmo AR, Wälchli T, Keller A, Carmeliet P, De Bock K.
Role of the GLUT1 Glucose Transporter in Postnatal CNS Angiogenesis and Blood-Brain Barrier Integrity.
Circ Res. 2020 Jul 31;127(4):466-482.
Eelen G*, Dubois C*, Cantelmo AR, Goveia J, Brüning U, DeRan M, Jarugumilli G, van Rijssel J, Saladino G, Comitani F, Zecchin A, Rocha S, Chen R, Huang H, Vandekeere S, Kalucka J, Lange C, Morales-Rodriguez F, Cruys B, Treps L, Ramer L, Vinckier S, Brepoels K, Wyns S, Souffreau J, Schoonjans L, Lamers WH, Wu Y, Haustraete J, Hofkens J, Liekens S, Cubbon R, Ghesquière B, Dewerchin M, Gervasio FL, Li X, van Buul JD, Wu X, Carmeliet P. *equal contribution
Role of glutamine synthetase in angiogenesis beyond glutamine synthesis.
Nature. 2018 Sep;561(7721):63-69.
Conradi LC*, Brajic A*, Cantelmo AR*, Bouché A, Kalucka J, Pircher A, Brüning U, Teuwen LA, Vinckier S, Ghesquière B, Dewerchin M, Carmeliet P. Tumor vessel disintegration by maximum tolerable PFKFB3 blockade.
Angiogenesis. 2017 Nov;20(4):599-613.
Cantelmo AR*, Conradi LC*, Brajic A*, Goveia J*, Kalucka J, Pircher A, Chaturvedi P, Hol J, Thienpont B, Teuwen LA, Schoors S, Boeckx B, Vriens J, Kuchnio A, Veys K, Cruys B, Finotto L, Treps L, Stav-Noraas TE, Bifari F, Stapor P, Decimo I, Kampen Kim, De Bock K, Haraldsen G, Schoonjans L, Rabelink T, Eelen G, Ghesquière, B, Rehman J, Lambrechts D, Malik AB, Dewerchin M, & Carmeliet P.
Inhibition of the glycolytic activator PFKFB3 in endothelial cells induces tumor vessel normalization, impairs metastasis and improves chemotherapy
Cancer Cell. 2016 Dec 12;30(6):968-985.
We are seeking a bioinformatician with expertise in multi-omics analysis, including metabolomics and spatial omics. To know more visit our LinkedIn profile (Sept 2025).
We are always interested in hosting Bachelor and Master students and welcome PhD applications from motivated candidates with a strong interest in vascular biology, endothelial cell plasticity, and related disease mechanisms.
Anna Rita CANTELMO
Institut Pasteur de Lille, U1011-EGID
1 rue du Professeur Calmette
59000 LILLE - France
e-mail: anna-rita.cantelmo[@]univ-lille.fr
office: +33 320 87 71 48
Alumni
Mauro Vedelago, PhD (2024-2025)
Fabiola Silva, PhD (2024-2025)
Mohamed Chamlali, PhD (2021-2023)
Wiliam Masselin (2022-2024)
Camille Dejos, PhD (2019-2021)
Mathilde Lebas, MSc (2018-2022)
Trainees
Mohamed Nasreldin, University of Lille, MSc training (2026)
Cyril Benda, University of Rennes, BSc training (2025)
Chamseddine Amarouche, University of Lille, BSc training (2025)
Segolene Tandrayen, University of Angers, BSc training (2025)
Alina Errerd, Erasmus +, German Cancer Research Center (DKFZ), MSc training (2024)
Camille Lafeuille, Apprentis Chercheur, high school student (2023)
Victorea Banza, University of Lille, BSc training (2023)
Evan Courmont, University of Lille, MSc training (2023)
Louay Bettieb, University of Lille, MSc training (2022)
Afaf Ennahal, University of Lille, BSc training (2021)