Topic 3: Immunometabolic dialogue in obesity and its comorbidities

Presentation of the research topic
Obesity has risen to reach epidemic proportions world-wide and predisposes to metabolic dysregulations, hence precipitating diseases such as dyslipidemia, MASLD and T2D. These metabolic disorders increase the risk for cardiovascular complications due to a combination of atherosclerosis of the coronary arteries and altered heart muscle and valve function, a condition often referred to as cardiometabolic disease. Cardiometabolic diseases are increasingly recognized to involve altered multi-organ interactions. Importantly, alterations in immune-inflammatory functions, the main focus of team 3, contribute to increase the cardiovascular risk and recent evidence has emerged showing that improper metabolic control acts in concert with immune system alterations to precipitate disease progression. It is thus important not only to study the role of individual organs in cardiometabolic disease, but also to study the role of altered inter-organ crosstalk including the key contribution of the immune system in this process. Thus, Team 3 studies the mechanisms of bidirectional crosstalk between the immune-inflammatory system and metabolism, in particular in the context of “metabolic” diseases: obesity, type 2 diabetes (T2D), MASLD and their cardiovascular complications, and in “inflammatory” diseases: psoriasis. Team 3 uses different complementary approaches, from bench-to-bedside (pre-clinical mouse models, in vitro molecular studies, translational research) focusing on immune cells from different metabolic organs (liver, heart, adipose tissues) as well as from their “classical” location in blood, bone marrow and barrier organs such as skin. Successful accomplishment of these objectives requires pluri-disciplinary competences and translational approaches. Therefore, Team 3 has developed its projects in close interaction with the other teams of the unit, allowing whole organism/systems biology approaches.
Key-words
Inflammation ; Immune Cells ; Nuclear receptors ; Type 2 Diabetes ; Obesity, Atherosclerosis ; MASH ; MASLD ; Psoriasis ; Atopic Dermatitis ; Allergic Asthma
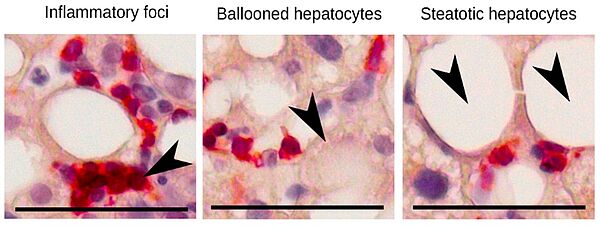
Presence of CD8 cytotoxic lymphocytes within inflammatory foci and in the vicinity of ballooned and steatotic hepatocytes. in the liver of a patient with Metabolic-associated SteatoHepatitis (MASH) (from Haas et al. Nat Metab. 2019)
David DOMBROWICZ
Team Leader. Research Director. INSERM
david.dombrowicz[@]inserm.fr david.dombrowicz[@]pasteur-lille.fr david.dombrowicz[@]univ-lille.fr
ORCID Number : 0000-0002-0485-8923
Jonas SONDERGAARD
Emerging Group Leader. Institut Pasteur de Lille.
jonas.sondergaard[@]pasteur-lille.fr
ORCID Number : 0000-0002-4438-6756
Sebastien FLEURY
Engineer. INSERM
sebastien.fleury[@]pasteur-lille.fr
ORCID Number :0000-0002-0679-3086
Zouriatou GOUDA
Engineer. Lille University
zouriatou.gouda[@]pasteur-lille.fr
ORCID Number. 0000-0001-5562-9322
Sandrine QUEMENER
Engineer. Lille University
sandrine.quemener[@]univ-lille.fr
ORCID Number : 0000-0001-6369-3906
01.01.2025. Arrival of Dr Jonas Sondergaard, leader Emerging Group Institut Pasteur de Lille. Role of Human Immune System Heterogeneity in Metabolic-dysfunction Associated Steatohepatitis (MASH) for Personalized Medicine.
01.01.2025. Arrival of Dr Janyerke Tulyeu, post-doctoral fellow. (coll. B Staels & D. Montaigne). Liver-induced Immune-mediated regulation of Cardiac Remodeling
- Preclinical diseases models: Type 2 Diabetes, Obesity, Atherosclerosis, MASH, Food Allergy, Colitis, Allergic Asthma, Atopic Dermatitis, Psoriasis, Contact hypersensibility
- Translational studies (collaborations with Pr. D.Staumont-Sallé, Département de Dermatologie, Hôpital Universitaire de Lille, Pr. F. Pattou, Inserm U1190 et Département de Chirurgie Métabolique, Hôpital Universitaire de Lille et Pr. S. Francque, Département d’hépatologie, AZ Antwerpen (Belgique)).
- Cytometry: Spectral cytometry, Mass cytometry, FACS
- Metabolic Immunophenotyping platform: www.egid.fr/plateformes/
- Invasive plethysmography
- Histology: Immunohistochemistry, Laser microdissection
- Intracellular metabolism: Oxymetry by “Seahorse” or SCENITH
- Genetically modified murine model
- Molecular biology: (RT-PCR, microarrays).
List of fundings between 2018 and 2023
European and international grants
2021 National Psoriasis Foundation (USA). Role of Pentose Phosphate Pathway in Dendritic cell migration in Psoriasis (38 000$) (coordinator).
2016-2022 European Research Council. Advanced. Bile acid, immune-metabolism, lipid and glucose homeostasis (2 500 000 € total ca. 800 000 € for Team 3). (Partner, Head immunology).
2017-2020 Francophone Fondation for Research on Diabetes (FFRD). Role of Treg-expressed RORα in type2 diabetes (coordinator) (300 000 €).
2017-2022 Leducq Foundation. https://www.fondationleducq.org/network/lean-leducq-epigenetics-of-atherosclerosis-network-defining-and-targeting-epigenetic-pathways-in-monocytes-and-macrophages-that-contribute-to-cardiovascular-disease. (500 000 $ total ca. 100 000 € for Team 3). (Partner, Immunology).
National public grants
2018-2023 National Research Agency. Generic Program. Contribution of ILC and CD8 to Non-Alcoholic Steatohepatitis and progression towards hepatocarcinoma (partner) (183 000 €).
2021-2025 National Research Agency. Generic Program. Role of humanized IgE and FceRI in asthma and its potentiation by obesity (partner) (250 000 €).
2022-2026 National Research Agency. Generic Program. Metabolic control of IL-23 by resident and migratory dendritic cells (coordinator) (310 000 €).
2023-2027 National Research Agency. Generic Program. Defining adipose tissue macrophage diversity and function (partner) (150 000 €).
2023-2027 National Research Agency. Generic Program. Liver-induced immune-mediated regulation of cardiac remodeling (partner) (100 000 €).
PIA (National excellence program) grants
2010-2024 National Research Agency. Labex EGID. (European Genomic Institute of Diabetes). (Partner, Head of Axis 5. adipose tissue immune cells) (38 000 000 € total, ca. 1 000 000 € for Team 3).
2016-2021 National Research Agency. RHU PreCINASH. (Partner, Immunology) (1 800 000 € total).
2020-2021 iSite Université de Lille. Contribution of metainflammation to COVID 19 pneumonia in obese patients (coordinator) (79 000 €).
Grants from foundations and charities
2018-2021 Foundation for Medical Research (FRM). Cardiovascular diseases. Role of myeloid-expressed RORα in atherosclerosis (coordinator) (300 000 €).
2022-2025 Foundation for Medical Research (FRM). Equipe FRM Liver-induced immune-mediated regulation of cardiac remodeling (partner) (100 000 €) (362 000 € total, ca. 120 000 € for Team 3).
2023-2026 Foundation for Medical Research (FRM). Equipe FRM Regulation of dendritic cell function in psoriasis: the hexosamine biosynthetic pathway at the crossroad between glucose and glutamine metabolism (coordinator) (376 000 €).
5 major publications
- L'Homme, L., B.P. Sermikli, J. Haas, S. Quemener, S. Fleury, V. Guinot, E. Barreby, N. Esser, J. Noulette, B. Derudas, B. Legendre, V. Reverdy, N. Paquot, J. PIette, S. Legrand-Poels, M. Aouadi, F. Pattou, B. Staels, and D. Dombrowicz. 2024. Selective Immune and non-immune contribution to GDF-15 production in obesity, type 2 diabetes and NASH. Nature communications 15:7173. https://www.nature.com/articles/s41467-024-51078-2. Study of the source of satiety regulator GDF-15 production over the progressive development of obesity, type 2 diabetes and MASLD/MASH. Recruitment of inflammatory macrophages into adipose tissue is the main GDF-15 in obesity while stressed hepatocytes become the predominant source with the development of MASLD/MASH
- Ninni, S*., D. Dombrowicz*, T. Kuznetsova, R. Vicario, V. Gao, O. Molendi-Coste, J. Haas, E. Woitrain, A. Coisne, A.E. Neele, K. Prange, L. Willemsen, S. Aghezzaf, S. Fragkogianni, A. Tazibet, L. Pineau, J.R. White, J. Eeckhoute, M. Koussa, H. Dubrulle, F. Juthier, J. Soquet, A. Vincentelli, J.L. Edme, M. de Winther, F. Geissmann, B. Staels, and D. Montaigne. 2023. Hematopoietic Somatic Mosaicism Is Associated With an Increased Risk of Postoperative Atrial Fibrillation (* Equal Contribution). J Am Coll Cardiol 81:1263-1278. With editorial material. (Top 5% cited). https://www.sciencedirect.com/science/article/pii/S073510972300267X?via%3Dihub. Demonstration of the impact of hematopoietic somatic mutations on early and late postoperative events following aortic valve replacement surgery and correlation with preoperative immune status investigated using mass cytometry and RNAsequencing.
- D.A. Mogilenko, J.T. Haas, L. Lhomme, S. Fleury, S. Quemener, M. Levavasseur, C. Becquart, J. Wartelle, A. Bogomolova, L. Pineau, O. Molendi-Coste, S. Lancel, H. Dehondt, C. Gheeraert, A. Melchior, C. Dewas, A. Nikitin, S. Pic, N. Rabhi, J.S. Annicotte, S. Oyadomari, T. Velasco-Hernandez, J. Cammenga, M. Foretz, B. Viollet, M. Vukovic, A. Villacreces, K. Kranc, P. Carmeliet, G. Marot, A. Boulter, S. Tavernier, L. Berod, M.P. Longhi, C. Paget, S. Janssens, D. Staumont-Salle, E. Aksoy, B. Staels, D. Dombrowicz. Metabolic and innate immune cues merge into a specific inflammatory response via the UPR. Cell, 2019, 177, 1201-1216 (With editorial material. Avancée de l’Inserm 2019 (Top 5% cited). Mechanistic demonstration of the exacerbation of innate (TLR- and non TLR-mediated) immune response by fatty acids through inhibition of glycolysis, increased mtROS production and syngerstici activation of UPR with IL-23 as a signature hallmark. Therapeutic applications for psoriasis.
- J.T. Haas, L. Vonghia, D.A.Mogilenko, A. Verrijken, O. Molendi-Coste, S. Fleury, A. Deprince, A. Nikitin, E. Woitrain, L. Ducrocq-Geoffroy, S. Pic, B. Derudas, H. Dehondt, C. Gheeraert, L. Van Gaal, A. Driessen, P. Lefebvre, B. Staels, S. Francque, D. Dombrowicz. Transcriptional network analysis implicates altered hepatic immune function in NASH development and resolution. Nat. Metab., 2019, 1, 604-614 https://www.nature.com/articles/s42255-019-0076-1 With editorial material. (Top 5% cited). Demonstration of the importance of the DC-CD8 axis to NASH development in humans and in preclinical model.
- R. Paumelle, J.T. Haas, N. Hennuyer, E. Bauge, Y. Deleye, D. Mesotten, L. Langouche, J. Vanhoutte, C. Cudejko, K Wouters, S.A. Hannou, V. Legry, S. Lancel, F. Lalloyer, A. Polizzi, S. Smati, P. Gourdy, E. Vallez, E. Bouchaert, B. Derudas, H. Dehondt, C. Gheeraert, S. Fleury, A. Tailleux, A. Montagner, W. Wahli, G. Van Den Berghe, H. Guillou, D. Dombrowicz*, B. Staels*. (*equal contribution). Hepatic PPARα is critical in the metabolic adaptation to sepsis. J. Hepatol, 2019, 70, 963-973 (Top 10% cited). Demonstration of the key role of hepatocyte-expressed rather than immune-expressed PPARa in sepsis outcome through regulation of glycaemia and ketogenesis rather than through an anti-inflammatory effect.
For a complete list of publications of team 3
David DOMBROWICZ
Team Leader. Research Director INSERM
david.dombrowicz[@]inserm.fr ; david.dombrowicz[@]pasteur-lille.fr ; david.dombrowicz[@]univ-lille.fr
Postal address. Inserm U1011. Institut Pasteur de Lille. 1, r. Prof. Calmette BP245. 59019 Lille Cedex. France


